Philippine Vaccine Effectiveness Study: A Post-COVID-19 Vaccination Surveillance Among Filipino Adults
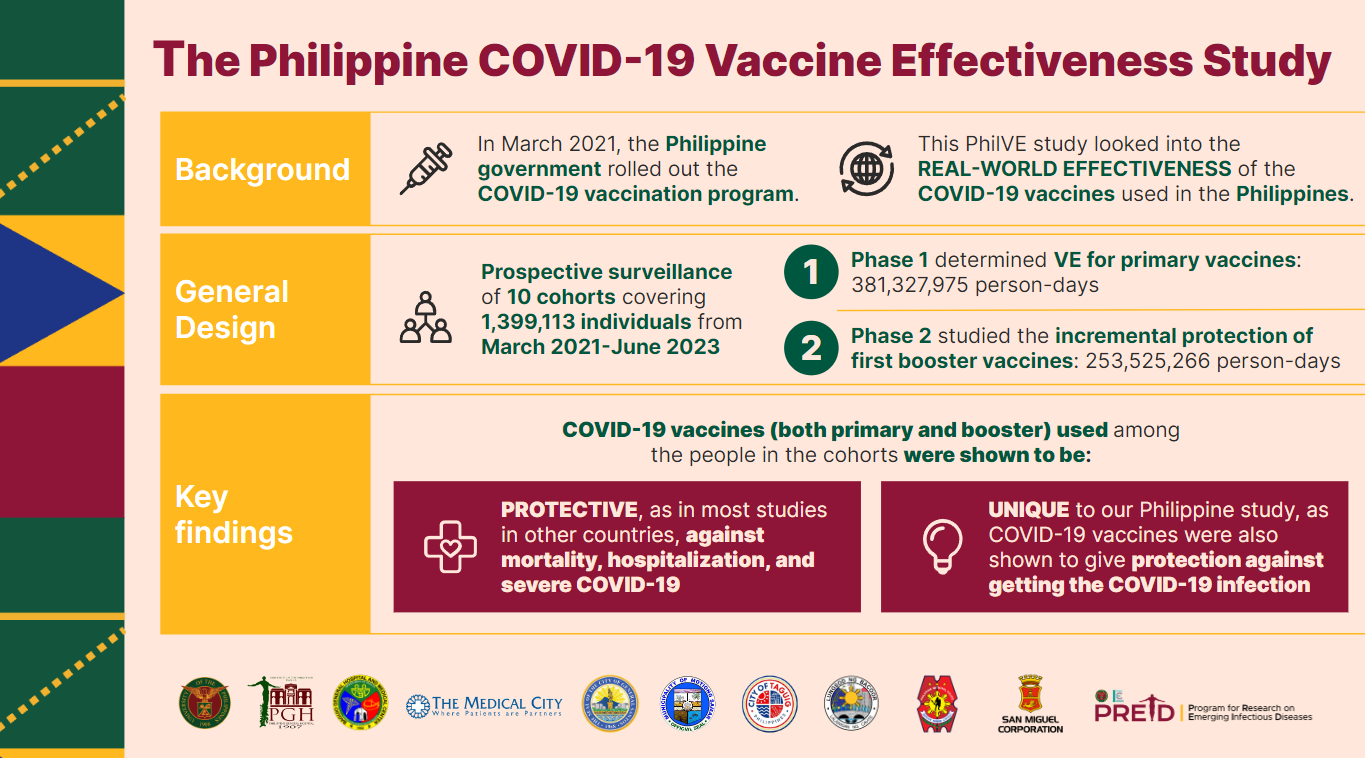
The Philippine Vaccine Effectiveness Study (PhilVE) is the inaugural project under the Program for Research on Emerging Infectious Diseases (PREID). PhilVE estimates the effectiveness of the COVID-19 vaccines used in the Philippines by immunogenicity and prevention of symptomatic COVID-19. Ten cohort sites, where the National COVID-19 Vaccination Program was rolled out, were engaged for 12 months for this research.
The PhilVE study is conducted under the University of the Philippines Manila through the National Institutes of Health – Institute of Clinical Epidemiology (ICE) and is funded by the Department of Science and Technology – Philippine Council for Health Research and Development (DOST-PCHRD).
Why PhilVE Matters
The Philippine Vaccine Effectiveness (PhilVE) project aims to shed light on knowledge gaps on:

- Antibody immune response from vaccination
- Understanding detection and recognition of antibody adaptive immune responses after vaccination
- Population and individual variations in immune response which affect vaccine effectiveness in different subgroups defined by factors such as age, sex, comorbidities
- Determine the duration of protection through serial antibody testing
- Early recognition of emergence of new coronavirus strains among those vaccinated.
PhilVE Study Objectives:

❶
Determine the specific IgG to SARS-CoV-2 levels obtained at 2, 12, 24, 36, and 52 weeks after the second dose among adults who received any of the EUA-approved COVID-19 vaccines
❷
Compare the IgG levels obtained at 2, 12, 24, 36, and 52 weeks after second dose according to type of vaccine received, age, sex, comorbidities
❸
Describe the COVID-19 infections over a 12-month period from the second dose of COVID-19 vaccines which will occur among vaccinated and unvaccinated individuals within the clusters including identification of variants of concern as the etiologies of such post vaccination COVID-19 infections.